SUFFERING FROM ANY KIND OF DISEASES?
Premilife gives energy to the human body that increases the chances of winning diseases (which diseases? more than 700!) - the means - homeopathic remedies
Over 700 Homeopathic Remedies - Enjoy the Benefits of Our Treatments
Share
this
Recent events have shown us (again) how rapidly a new disease can take root and spread. Such events are accompanied by an explosion of clinical and epidemiological information and research. The goal of Premilife is to open whatever resources we can to help our communities, researchers and clinicians contain and manage this disease. Our resources span scientific and medical journals and textbooks, educational products, and a variety of other resources, like travel precautions from and media posts of interest to ours communities.
Premilife is a professional homeopathic remedies company dedicated to offering a wide range of complex homeopathic remedies for different diseases. Two-thirds of all diseases in the world are either still not treated adequately or not treated at all.
We believe in Integrative medicine – Rather than one practice care model, integrative health combines alternative care, western medicine, eastern medicine and complementary medicine to achieve the best possible results for the patient.
Treatments success and scientific breakthroughs are more likely to occur when scientist are free to tackle problems from different angles and ways.
Most people who catch the new coronavirus SARS-CoV-2 recover at home, and some need hospitalization to fight the virus. But in a number of patients, the disease called COVID-19 is deadly. Some people are at higher risk of getting very sick from this illness. It includes older adults, people who have serious chronic medical conditions, people with compromised immune systems, people having chemotherapy or who have received chemotherapy in the last three months and people having immunotherapy, among others.
A coronavirus pandemic can overwhelm the capacity of outpatient facilities, emergency departments (EDs), hospitals, and intensive care units, leading to critical shortages of staff, space, and supplies with serious implications for patient outcomes.
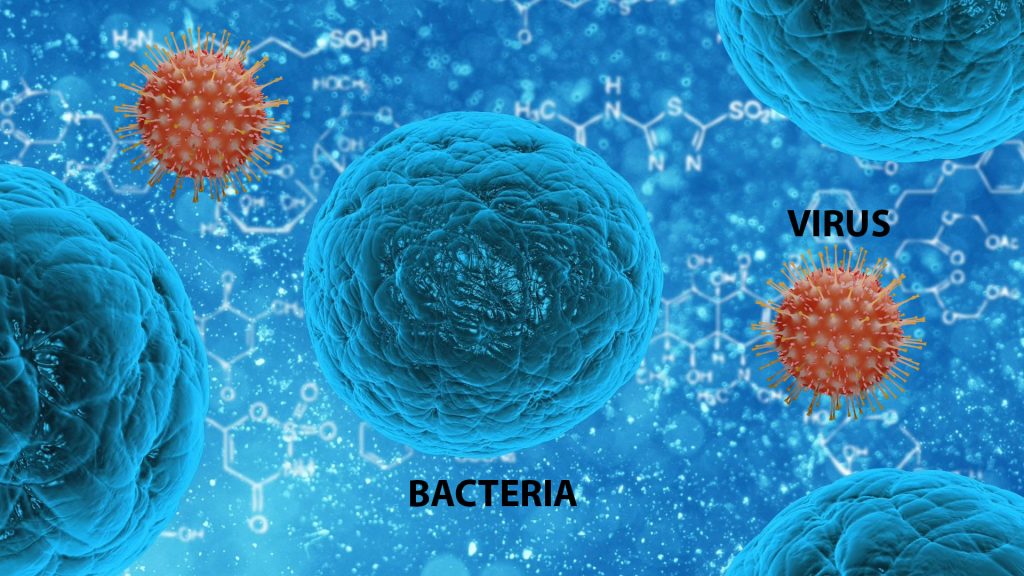
Bacteria
Living Cells, size more than 1000nm
Need moisture
Most need oxygen
Need Nutrients to reproduce
Growth by cell division
Some can cell propel
Viruses
Not living cell - Inert DNA/RNA in a protein shell, size more than 100nm.
Are the most numerous type of biological entity.
Infect all types of life forms.
Replicates only inside the living cells.
Do not need moisture or oxygen.
Do not need nutrients to reproduce and not growth by cell division.
Do not self-propel.
Increases genetic diversity by horizontal gene transfer (the movement of genetic material between unicellular and/or multicellular organisms other than by the ("vertical") transmission of DNA from parent to offspring (reproduction).
Bacterial and viral infections have many things in common. Both types of infections are caused by microbes – bacteria and viruses, respectively – and spread by things such as:
Bacteria and viruses are too tiny to be seen by the naked eye, can cause similar symptoms and are often spread in the same way, but that’s where the similarities end.
Bacteria are relatively complex, single-celled creatures, many with a rigid wall, and a thin, rubbery membrane surrounding the fluid inside the cell. They can reproduce on their own. Fossilized records show that bacteria have existed for about 3.5 billion years, and bacteria can survive in different environments, including extreme heat and cold, radioactive waste, and the human body.
Most bacteria are harmless, and some actually help by digesting food, destroying disease-causing microbes, fighting cancer cells, and providing essential nutrients. Fewer than 1% of bacteria cause diseases in people and are referred to as pathogenic bacteria.
Viruses are tinier: the largest of them are smaller than the smallest bacteria. All they have is a protein coat and a core of genetic material, either RNA or DNA.
Unlike bacteria, viruses are parasitic and require living hosts – such as people, plants or animals – to multiply. They can only reproduce by attaching themselves to cells. In most cases, they reprogram the cells to make new viruses until the cells burst and die. In other cases, they turn normal cells into malignant or cancerous cells.
Viruses may reproduce with fidelity or with errors (mutations); this ability to mutate is responsible for the ability of some viruses to change slightly in each infected person, making treatment difficult.
Also unlike bacteria, most viruses do cause disease, and they’re quite specific about the cells they attack. For example, certain viruses attack cells in the liver, respiratory system, or blood. In some cases, viruses target bacteria.
Viral infections in animals provoke an immune response that usually eliminates the infecting virus. Immune responses can also be produced by vaccines, which confer an artificially acquired immunity to the specific viral infection. Some viruses, including those that cause AIDS, HPV infection, and viral hepatitis, evade these immune responses and result in chronic infections.
Perhaps the most important distinction between bacteria and viruses is that antibiotic drugs usually kill bacteria, but they aren’t effective against viruses. Viral infections require either vaccinations to prevent them in the first place or antiviral drugs inhibit their development.
The coronaviruses include a large number of viruses that infect different animal species. The predominant diseases associated with these viruses are respiratory and enteric infections, although hepatic and neurological diseases also occur.
Coronaviruses have extraordinarily large single-stranded RNA genomes - approximately 27,000 to 34,000 bases or RNA "letters" in length, the largest among known RNA viruses.
Coronavirus particles are surrounded by a fatty outer layer called an envelope and usually appear spherical, as seen under an electron microscope, with a crown or "corona" of club-shaped spikes on their surface. These protrusions will only bind to certain receptors on the host cell; they are essential for both host specificity and viral infectivity.
Human coronaviruses were first discovered in the late 1960s.
Only 7 coronaviruses are known to cause disease in humans.
Four of the 7 coronaviruses most frequently cause symptoms of the common cold. Coronaviruses 229E and OC43 cause the common cold; the serotypes NL63 and HUK1 have also been associated with the common cold. Rarely, severe lower respiratory tract infections, including pneumonia, can occur, primarily in infants, older people, and the immunocompromised.
Three of the 7 coronaviruses cause much more severe, and sometimes fatal, respiratory infections in humans than other coronaviruses and have caused major outbreaks of deadly pneumonia in the 21st century:
The virus SARS-CoV2 primarily spreads between people who are in close contact with one another (within about 6 feet – 2 meters) in a similar way to influenza, via respiratory droplets from coughing or sneezing.
The time between exposure and symptom onset is typically five days, but may range from two to fourteen days.
Symptoms are most often fever, cough, and shortness of breath.
Complications may include:
a) Pneumonia, an infection that inflames the air sacs in one or both lungs. The air sacs may fill with fluid or pus (purulent material), causing cough with phlegm or pus, fever, chills, and difficulty breathing.
In one study of 138 patients hospitalized in Wuhan for pneumonia due to SARS-CoV-2, dyspnea developed after a median of five days since the onset of symptoms, and hospital admission occurred after a median of seven days of symptoms. In another study, the median time to dyspnea was eight days.
b) Acute respiratory distress syndrome (ARDS) is a rapidly progressive disease occurring in critically ill patients. The main complication in ARDS is that fluid leaks into the lungs making breathing difficult or impossible.
In one study of 138 patients, ARDS developed in 20 percent after a median of eight days, and mechanical ventilation was implemented in 12.3 percent. In another study of 201 hospitalized patients with COVID-19 in Wuhan, 41% percent developed ARDS; age greater than 65 years, diabetes mellitus, and hypertension were each associated with ARDS.
There is currently no vaccine or specific antiviral treatment, though research is ongoing. Efforts are aimed at managing symptoms and supportive therapy. Recommended preventive measures include handwashing, maintaining distance from people who are sick, and monitoring and self-isolation for fourteen days for people who suspect they are infected.
Public health responses around the world have included travel restrictions, quarantines, curfews, and school closures. They have included the quarantine of Hubei, China and Lombardy, Italy; various curfew measures in China; screening methods at airports and train stations; and travel advisories in regard to regions with ongoing community transmission, such as central China, South Korea, Italy, and Iran. Country wide school closures have occurred in several countries, and localized school closures in several others.
The wider impact of the outbreak includes social and economic instability, as well as xenophobia and racism against people of Chinese and East Asian descent, and the spread of misinformation and conspiracy theories about the virus online.

According to the World Health Organization (WHO), viral diseases continue to emerge and represent a serious issue to public health. On March 11, 2020, the World Health Organization declared the COVID-19 outbreak a pandemic.
We must realize that in our crowded world of 7.8 billion people, a combination of altered human behaviors, environmental changes, and inadequate global public health mechanisms now easily turn obscure animal viruses into existential human threats. We have created a global, human-dominated ecosystem that serves as a playground for the emergence and host-switching of animal viruses, especially genetically error-prone RNA viruses, whose high mutation rates have, for millions of years, provided opportunities to switch to new hosts in new ecosystems. It took the genome of the human species 8 million years to evolve by 1%. Many animal RNA viruses can evolve by more than 1% in a matter of days. It is not difficult to understand why we increasingly see the emergence of zoonotic viruses, meaning that they can be transmitted to people from animals.
The present outbreak of a coronavirus-associated acute respiratory disease called coronavirus disease 19 (COVID-19) is the third documented spillover of an animal coronavirus to humans in only two decades that has resulted in a major epidemic:
The earliest reported symptoms occurred on 1 December 2019, in a person who had not had any exposure to the Huanan Seafood Wholesale Market or to the remaining 40 of the first cluster detected with the new virus. Of this first cluster, two-thirds were found to have a link with the market, which also sold live animals.
The median age ranged from:
During the early stages, the number of cases doubled approximately every seven and a half days. In early and mid-January 2020, the virus spread to other Chinese provinces, helped by the Chinese New Year migration, as Wuhan is a transport hub in China and the infected individuals quickly spread throughout the country. On 20 January, China reported nearly 140 new patients in a day, including two people in Beijing and one in Shenzhen. Later official data shows that 6,174 COVID-19 virus-infected patients had already developed symptoms by 20 January 2020.
On 26 February 2020, WHO reported that, as new cases reported dropped in China but suddenly increased in Italy, Iran, and South Korea, the number of new cases outside China had exceeded the number of new cases in China for the first time on 25 February 2020.
Increasing numbers of cases have also been reported in other countries across all continents except Antarctica.
Situation update worldwide, as of 25 April 2020
Since 31 December 2019 and as of 25 April 2020, 2 744 744 cases of COVID-19 (in accordance with the applied case definitions and testing strategies in the affected countries)
have been reported, including 195 387 deaths.
Cases have been reported from:
Deaths have been reported from:
Up to date information – live, interactive map of global coronavirus cases.
Link: https://coronavirus.jhu.edu/map.html

Understanding of the transmission risk is incomplete.
People are infectious early in the course of disease.
Virus shedding continues for weeks after recovery-does not mean its infectious.
Truly asymptomatic are rare.
People are not getting reinfected after recovery.
Transmission in China mostly among family members and close contacts. The contribution of “community spread is unknow.
Covid-19 outbreak in China isn’t driven by spread in hospitals.
Covid-19 outbreak in China isn’t driven by spread in schools.
Still unknown true fatality rates.
A person is considered to be at risk of having COVID-19 if they have travelled to an area with ongoing community transmission within the previous fourteen days or has had close contact with an infected individual.
Although the outbreak is likely to have started from a zoonotic transmission event associated with a large seafood market that also traded in live wild animals, it soon became clear that efficient person-to-person transmission was also occurring.
The primary mode of transmission is via respiratory droplets.
Person-to-person spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is thought to occur mainly via respiratory droplets, resembling the spread of influenza. With droplet transmission, virus released in the respiratory secretions when a person with infection coughs, sneezes, or talks can infect another person if it makes direct contact with the mucous membranes. These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs. These only stay suspended in the air for a short time. Droplets typically do not travel more than six feet (about two meters) and do not linger in the air.
The virus is stable for several hours to days in aerosols and on surfaces, it was detectable in aerosols (a suspension of tiny particles or droplets in the air) for up to three hours, up to four hours on copper, up to 24 hours on cardboard and up to two to three days on plastic and stainless steel. Disinfection of surfaces is possible with substances such as 62–71% ethanol applied for one minute.
Some spread might be possible before people show symptoms; there have been reports of this occurring with this new coronavirus, but this is not thought to be the main way the virus spreads.
People are thought to be most contagious when they are most symptomatic (the sickest). Viral RNA levels appear to be higher soon after symptom onset compared with later in the illness.
According to a joint WHO-China report, the rate of secondary COVID-19 ranged from 1 to 5 percent among tens of thousands of close contacts of confirmed patients in China. In the United States, the symptomatic secondary attack rate was 0.45 percent among 445 close contacts of 10 confirmed patients.
How easily a virus spreads from person-to-person can vary. Some viruses are highly contagious (spread easily), like measles, while other viruses do not spread as easily. Another factor is whether the spread is sustained, spreading continually without stopping.
The virus that causes COVID-19 seems to be spreading easily and sustainably in the community (“community spread”) in some affected geographic areas.
SARS-CoV-2 RNA has been detected in blood and stool specimens. Live virus has been cultured from stool in some cases, but according to a joint WHO-China report, fecal-oral transmission did not appear to be a significant factor in the spread of infection
There have been estimates for the basic reproduction number (the average number of people an infected person is likely to infect), ranging from 2.13 to 4.82. As of 24 January 2020 the virus was reported to have been able to transmit down a chain of up to four people. This is similar to severe acute respiratory syndrome-related coronavirus (SARS-CoV).
Super-spreaders played an extraordinary role in driving the 2003 SARS outbreak and may also play a significant role in the current COVID-19 outbreak. A super-spreader is an individual who transmits an infection to a significantly greater number of other people than the average infected person.
The WHO furthermore writes that though history with SARS and MERS show that transmission through food does not happen, the possibility remains open and that meat and animal products should be cooked thoroughly as a rule of thumb (milk in developed countries is usually pasteurised).

Early information out of China, where COVID-19 first started, shows that some people are at higher risk of getting very sick from this illness. This includes:
A) Older adults: Most developed world countries have accepted the chronological age of 65 years as a definition of ‘elderly’ or older person, but like many westernized concepts, this does not adapt well to the situation in Africa. While this definition is somewhat arbitrary, it is many times associated with the age at which one can begin to receive pension benefits. At the moment, there is no United Nations standard numerical criterion, but the UN agreed cutoff is 60+ years to refer to the older population.
Older age was associated with increased mortality, with a case fatality rate of 8 and 15 percent among those aged 70 to 79 years and 80 years or older, respectively.
B) People who have serious chronic medical conditions like:
Many lung diseases involve a combination of these three types. Fatality rate 6 percent.
Lung disease and cardiac complications: Cardiac complications, including new or worsening heart failure, new or worsening arrhythmia, or myocardial infarction are common in patients with pneumonia. Cardiac arrest occurs in about 3% of inpatients with pneumonia. Risk factors of cardiac events after pneumonia include older age, pre-existing cardiovascular diseases, and greater severity of pneumonia at presentation. Coronary heart disease has also been found to be associated with acute cardiac events and poor outcomes in influenza and other respiratory viral infections.
Immune system, the complex group of defense responses found in humans and other advanced vertebrates that helps repel disease-causing organisms (pathogens). Immunity from disease is actually conferred by two cooperative defense systems, called nonspecific, innate immunity and specific, acquired immunity.
Viral Activation of Immunity Immunity to viral infection is caused by a variety of specific and nonspecific mechanisms. The activation of different immune functions and the duration and magnitude of the immune response depend on how the virus interacts with host cells (on whether it is a cytolytic, steady-state, latent, and/or integrated infection) and on how the virus spreads (by local, primary hematogenous, secondary hematogenous, and/or nervous system spread). Therefore, viral antigens may be present in different parts of the body depending on the route of spread and phase of infection. Local infections at surfaces such as the mucosa can elicit local cell-mediated and humoral (IgA) immune responses, but not necessarily systemic immunity. The host has multiple immune defense functions that can eliminate virus and/or viral disease.
Humoral Immunity: Virus and/or virus-infected cells can stimulate B lymphocytes to produce antibody (specific for viral antigens) Antibody neutralization is most effective when virus is present in large fluid spaces (e.g., serum) or on moist surfaces (e.g., the gastrointestinal and respiratory tracts). IgG, IgM, and IgA have all been shown to exert antiviral activity. Antibody can neutralize virus by: A) blocking virus-host cell interactions or B) recognizing viral antigens on virus-infected cells which can lead to antibody-dependent cytotoxic cells (ADCC) or complement-mediated lysis. IgG antibodies are responsible for most antiviral activity in serum, while IgA is the most important antibody when viruses infect mucosal surfaces.
Cell-Mediated Immunity: The term cell-mediated immunity refers to (A) the recognition and/or killing of virus and virus-infected cells by leukocytes and (B) the production of different soluble factors (cytokines) by these cells when stimulated by virus or virus-infected cells. Cytotoxic T lymphocytes, natural killer (NK) cells and antiviral macrophages can recognize and kill virus-infected cells. Helper T cells can recognize virus-infected cells and produce a number of important cytokines. Cytokines produced by monocytes (monokines), T cells, and NK cells (lymphokines) play important roles in regulating immune functions and developing antiviral immune functions.
Virus-Induced Immunopathology Immune-mediated disease may develop in certain virus infections in which viral antigens and uncontrolled immune hypersensitivity to them persist for a long period. Immune-mediated disease can be mediated by both humoral and cell-mediated immune functions. Immune-complex syndrome can be mediated by virus/virus antigen antibody complexes. T cells (cytotoxic and helper) can also mediate immunopathologic injuries via a number of mechanisms. Immunopathology can result from tissue/organ damage via cytotoxic T cells, inflammation induced via cytokines, antibody plus complement, antibody-antigen complexes and/or ADCC.
Roles of Immune Functions during Viral Infections The early, nonspecific responses (nonspecific inhibition, natural killer cell activity, and interferon) limit virus multiplication during the acute phase of virus infections. The later specific immune (humoral and cell-mediated) responses function to help eliminate virus at the end of the acute phase, and subsequently to maintain specific resistance to reinfection.
COVID-19
Cytopathic viruses, including SARS-CoV-2, induce death and injury of virus-infected cells and tissues as part of the virus replicative cycle.
People with compromised immune system are at a higher risk from the coronavirus. There are many things that can cause a weak immune system (immunosuppressed). These include: cancer treatment, treatment for autoimmune diseases, such as rheumatoid arthritis, lupus, multiple sclerosis (MS) and inflammatory bowel diseases, HIV, having an organ transplant or a bone-marrow transplant.
Recent studies have shown that in addition to dyspnea, hypoxemia, and acute respiratory distress, lymphopenia, and cytokine release syndrome are also important clinical features in patients with severe SARS-CoV-2 infection. This suggests that homeostasis of the immune system plays an important role in the development of COVID-19 pneumonia.
SARS-CoV-2 infection and the destruction of lung cells triggers a local immune response, recruiting macrophages (a type of white blood cell of the immune system that engulfs and digests cellular debris, foreign substances, microbes, cancer cells, and anything else that does not have the type of proteins specific to healthy body cells on its surface) and monocytes that respond to the infection, release cytokines and prime adaptive T and B cell immune responses (Both T and B cell responses against SARS-CoV-2 are detected in the blood around 1 week after the onset of COVID-19 symptoms).
B cell responses typically arise first against the nucleocapsid (N) protein. Within 4–8 days after symptom onset, antibody responses to S protein are also found.
In most individuals, recruited cells clear the infection in the lung, the immune response recedes and patients recover. However, in some patients, a dysfunctional immune response occurs, which triggers a cytokine storm that mediates widespread lung inflammation.
The mechanisms by which SARS-CoV-2 subverts the body’s innate antiviral cytokine responses are yet to be studied, but research on SARS-CoV shows that multiple viral structural and non-structural proteins antagonize interferon responses. Antagonism occurs at various stages of the interferon signalling pathway, including by preventing PRR recognition of viral RNA.
COVID-19 patients, especially those with severe infection, showed increased levels of regulatory molecules and decreased levels of multiple cytokines in peripheral blood T cells.
Very high levels of cytokines and chemokines in the blood and lungs of SARS patients were reported on autopsy. Protein or RNA expression of IL-6, CCL2/MCP-1, and CXCL10/IP-10 was detected by IHC and RT-PCR in the lungs of patients with fatal SARS cases. Elevation of IL-6 and IL-8 during the acute phase of disease and persistent expression of CCL2/MCP-1, CXCL9/MIG, and CXCL10/IP-10 in both acute and fatal cases were consistently detected in the blood of SARS patients. Moreover, increased serum levels of CXCL10/IP-10, IL-2, and IL-6 correlated significantly with SARS pneumonitis in patients.
Chemokine receptors play a crucial role in directing inflammatory cells to the sites of infection. Previous studies have documented the importance of chemokine receptors in protection from SARS-associated disease. CCR1-, CCR2-, and CCR5-deficient mice developed severe disease and mortality without inflammatory cell recruitment to the lungs. In contrast, upregulation of chemokines MCP-1/CCL2 (CCR1), MIP-1α/CCL3 (CCR2), and RANTES/CCL5 (CCR5) in the lungs of wild-type mice coincided with pulmonary recruitment of inflammatory cells and protected the mice from mortality. The protective role of CXCR3 (CXCL9/MIG and CXCL10/IP-10) in cell-mediated viral clearance of West Nile virus (WNV) infection has been documented, where CXCR3-deficient mice exhibited significantly enhanced mortality.
In an experiments with SARS-CoV infection in senescent mice, the biphasic increased expression of chemokines (MCP-1/CCL2, MIP-1α/CCL3, RANTES/CCL5, MIG/CXCL9, and IP-10/CXCL10) and their receptors (CCR2, CCR5, and CXCR3) is coincident with the appearance of inflammatory cells in the lungs.
Cytotoxic lymphocytes such as cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are necessary for the control of viral infection, and the functional exhaustion of cytotoxic lymphocytes is correlated with disease progression.
CD8+ T cells are important for directly attacking and killing virus-infected cells,
whereas CD4+ T cells are crucial to prime both CD8+ T cells and B cells; and are also responsible for cytokine production to drive immune cell recruitment.
The total number of NK and CD8+ T cells was decreased markedly in patients with SARS-CoV-2 infection. The function of NK and CD8+ T cells was exhausted with the increased expression of NKG2A in COVID-19 patients. Importantly, in patients convalescing after therapy, the number of NK and CD8+ T cells was restored with reduced expression of NKG2A. These results suggest that the functional exhaustion of cytotoxic lymphocytes is associated with SRAS-CoV-2 infection. Hence, SARS-CoV-2 infection may break down antiviral immunity at an early stage.
Nearly everyone who recovers from COVID-19 makes antibodies against the new coronavirussuggesting that they are immune from reinfection for an unknown length of time. The immune response could be slow and some patients didn’t produce detectable antibodies until one month after they first started feeling ill.
Controlling the inflammatory response may be as important as targeting the virus.
People with cancer might be more likely to get infections because of:
The cancer itself
Certain types of cancer treatment
Poor nutrition
Other health problems or medications that aren't related to cancer
Cancer patients are among those at high risk of serious illness from an infection because their immune systems are often weakened by cancer and its treatments. Most people who were treated for cancer in the past (especially if it was years ago) are likely to have normal immune function, but each person is different. It’s important that all cancer patients and survivors, whether currently in treatment or not, talk with a doctor who understands their situation and medical history.
This is because some treatments, like chemotherapy, can stop the bone marrow from making enough white blood cells. White blood cells are part of your immune system. This is most likely to happen during a course of cancer treatment, but the effects can last for some time afterwards.
Some types of cancer can also lower your ability to fight infection. This is usually cancer that affects your immune system like leukaemia or lymphoma.
The effects of coronavirus infection could be more severe for certain people with prostate cancer. These include:
When your ability to fight infection is lowered the symptoms of any infection can be much more severe and may become dangerous.
Infectious diseases are the second-leading cause of mortality in patients with cancer, whose immune systems are often compromised. While less than 1 percent of people who were otherwise healthy died from COVID-19, the fatality rate for people with cardiovascular disease was 10.5 percent. That figure was 7.3 percent for diabetes patients and around 6 percent for those with chronic respiratory disease, hypertension, or cancer.
HIV
At the present time, we have no specific information about the risk of COVID-19 in people with HIV.
The risk from immune suppression is not known, but with other viral respiratory infections, the risk for people with HIV getting very sick is greatest in:
People with a low CD4 cell count, and
People not on HIV treatment (antiretroviral therapy or ART).
People with HIV can also be at increased risk of getting very sick with COVID-19 based on their age and other medical conditions.
Some types of HIV medicine (in particular, lopinavir/ritonavir) are being evaluated in clinical trialsexternal icon to treat COVID-19. While there is some evidence that this type of HIV medicine might help treat infections with SARS and MERS (two other coronaviruses related to the virus that causes COVID-19), there are no data available yet from clinical trials that these drugs help people with COVID-19.
People with HIV should not switch their HIV medicine in an attempt to prevent or treat COVID-19.
If a COVID-19 outbreak happens in your community, it could last for a long time (an outbreak is when a large number of people suddenly get sick.) Depending on how severe the outbreak is, public health officials may recommend community actions to reduce people’s risk of being exposed to COVID-19. These actions can slow the spread and reduce the impact of disease.
If you are at higher risk for serious illness from COVID-19 because of your age or because you have a serious long-term health problem, it is extra important for you to take actions to reduce your risk of getting sick with the disease.
The SARS-CoV-2 coronavirus produces the same clinical symptoms in pregnant women as it does other infected people, and there is currently no evidence for vertical transmission.
Traditionally, it has been suggested that pregnancy causes an immunosuppressive state that would facilitate fetal tolerance and result in an increased susceptibility to infection.
Pregnant women are particularly susceptible to respiratory pathogens and severe pneumonia, because they are at an immunosuppressive state, and physiologic adaptive changes during pregnancy (e.g., diaphragm elevation, increased oxygen consumption, and edema of respiratory tract mucosa) render them intolerant to hypoxia.
Previous epidemics of many emerging viral infections have typically resulted in poor obstetrical outcomes including maternal morbidity and mortality, maternal-fetal transmission of the virus, and perinatal infections and death.
We do not currently know if pregnant women have a greater chance of getting sick from COVID-19 than the general public nor whether they are more likely to have serious illness as a result. Pregnant women experience changes in their bodies that may increase their risk of some infections. With viruses from the same family as COVID-19, and other viral respiratory infections, such as influenza, women have had a higher risk of developing severe illness.
Literature describing 38 pregnant women with COVID-19 and their newborns in China to assess the effects of SARS-CoV-2 on the mothers and infants including clinical, laboratory and virologic data, and the transmissibility of the virus from mother to fetus reveals that unlike coronavirus infections of pregnant women caused by SARS and MERS, in these 38 pregnant women COVID-19 did not lead to maternal deaths.
At this point in the global pandemic of COVID-19 infection there is no evidence that SARS-CoV-2 undergoes intrauterine or transplacental transmission from infected pregnant women to their fetuses. Analysis of additional cases is necessary to determine if this remains true.
Any pregnant woman who has travelled in a country affected by SARS-CoV-2 within the previous 14 days or who has had close contact with a patient with confirmed SARS-CoV-2 infection should be tested with a SARS-CoV-2 nucleic acid amplification test, even if asymptomatic.
Pregnant women with laboratory-confirmed SARS-CoV-2 infection who are asymptomatic should be self-monitored at home for clinical features of COVID-19 for at least 14 days. These patients and those recovering from mild illness should be monitored with bimonthly fetal growth ultrasounds and Doppler assessments because of the potential risk for intrauterine growth restriction. Pregnant women with COVID-19 pneumonia should be managed by a multidisciplinary team at a tertiary care center. When quick Sepsis-related Organ Failure Assessment criteria are met, the patient should be transferred to an intensive care unit.
For pregnant women with confirmed infection, the choice of delivery timing should be individualised depending on the week of gestation and maternal, fetal, and delivery conditions. Whenever possible, vaginal delivery via induction of labour, with eventual instrumental delivery to avoid maternal exhaustion, should be favoured to avoid unnecessary surgical complications in an already sick patient. Septic shock, acute organ failure, or fetal distress should prompt emergency cesarean delivery (or termination if legal before fetal viability).
Newborns of mothers positive for SARS-CoV-2 should be isolated for at least 14 days or until viral shedding clears, during which time direct breastfeeding is not recommended.
In limited studies on women with COVID-19 and another coronavirus infection, Severe Acute Respiratory Syndrome (SARS-CoV), the virus has not been detected in breast milk; however we do not know whether mothers with COVID-19 can transmit the virus via breast milk.
The term mortality comes from the Latin word mortalitas. The mortality rate is the number of deaths within a given population in a given time period. The mortality rate is typically expressed in number of deaths per 1,000 or 100,000 individuals per year.
Definition of death-to-case ratio
The death-to-case ratio is the number of deaths attributed to a particular disease during a specified time period divided by the number of new cases of that disease identified during the same time period. The death-to-case ratio is a ratio but not necessarily a proportion, because some of the deaths that are counted in the numerator might have occurred among persons who developed disease in an earlier period, and are therefore not counted in the denominator.
The maximum incubation period is assumed to be up to 14 days, whereas the median time from onset of symptoms to intensive care unit (ICU) admission is around 10 days. Recently, WHO reported that the time between symptom onset and death ranged from about 2 weeks to 8 weeks.
Risk factors assessment associated with death in adults during the stay in an intensive care unit (ICU).
Specifically, a) being of an older age, b)showing signs of sepsis, and c) having blood clotting issues when admitted to hospital are key risk factors associated with higher risk of death from the new coronavirus.
a) Being of older age, increased age was associated with death in patients with COVID-19. The age-dependent defects in T-cell and B-cell function and the excess production of type 2 cytokines could lead to a deficiency in control of viral replication and more prolonged proinflammatory responses, potentially leading to poor outcome.
b) Showing signs of sepsis, a severe condition resulting from the presence of harmful microorganisms in the blood or other tissues and the body’s response to their presence, potentially leading to the malfunctioning of various organs, shock, and death.
Sepsis is most common and most dangerous in: Older adults, Pregnant women, Children younger than 1, People who have chronic conditions, such as diabetes, kidney or lung disease, or cancer and People who have weakened immune systems.
Sepsis Symptoms:
Severe sepsis occurs when there’s organ failure. You must have one or more of the following signs to be diagnosed with severe sepsis:
Septic shock
Symptoms of septic shock include the symptoms of severe sepsis, plus a very low blood pressure – the organs of the body fail to receive sufficient oxygen. To be diagnosed with septic shock, you must have a probable or confirmed infection and both of the following:
c) Having blood clotting issues
Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia.
In patients with COVID-19 infection, the development of 1) coagulopathy and 2) disseminated intravascular coagulation (DIC) appears to be associated with a high mortality rate.
The potential risk factors of older age, 3) high SOFA score, and 4) d-dimer greater than 1 μg/mL could help clinicians to identify patients with poor prognosis at an early stage.
The first confirmed death was on 9 January 2020 in Wuhan. The first death outside China occurred in the Philippines, and the first death outside Asia was in France. As of 13 March 2020, outside of mainland China, more than a dozen deaths have been recorded in each of Iran, South Korea, and Italy. Deaths have also been reported in North America, Australia, San Marino, Spain, Iraq and the United Kingdom.
As of 3 March 2020, WHO data shows the percentage of infected people who have died from COVID-19 is 3.4% globally (1.6% outside of China).
As of March 21, 2020 – Total death in most affected countries:
| Country | Total Death |
| Italy | 4,032 |
| China | 3,255 |
| Iran | 1,556 |
| Spain | 1,378 |
| France | 450 |
| USA | 279 |
| UK | 177 |
| Netherlands | 136 |
| S. Korea | 102 |
COVID-19 Fatality Rate by AGE:
The CDC’s analysis found that 31 percent of all cases, 45 percent of hospitalizations, 53 percent of ICU admissions and 80 percent of all fatalities from the virus occurred among adults aged 65 plus with the highest percentage of severe outcomes happening among those aged 85 and over.
Fatality rate males vs females
COVID-19 shows a difference in fatality rate between males (2.8%) and females (1.7%).
As ACE2 is located on the X chromosome, there may be alleles that confer resistance to COVID-19, explaining the lower fatality rate in females. Alternatively, the oestrogen and testosterone sex hormones have different immunoregulatory functions, which could influence immune protection or disease severity.
The proportion of severe or fatal infections may vary by location. As an example, in Italy, 12 percent of all detected COVID-19 cases and 16 percent of all hospitalized patients were admitted to the intensive care unit; the estimated case fatality rate was 5.8 percent in mid-March. In contrast, the estimated case fatality rate in mid-March in South Korea was 0.9 percent. This may be related to distinct demographics of infection; in Italy, the median age of patients with infection was 64 years, whereas in Korea the median age was in the 40s.
Up to date information – live, interactive map of global coronavirus cases.
Link: https://coronavirus.jhu.edu/map.html

Genome: In the fields of molecular biology and genetics, a genome is the genetic material of an organism.
Encode: A gene is a genomic sequence (DNA or RNA) directly encoding functional product molecules, either RNA or protein. In the case that there are several functional products sharing overlapping regions, one takes the union of all overlapping genomic sequences coding for them.
Polymerase chain reaction (PCR) a revolutionary method developed by Kary Mullis in the 1980s, is widely used in molecular biology to rapidly make millions to billions of copies of a specific DNA sample allowing scientists to take a very small sample of DNA and amplify it to a large enough amount to study in detail.
The nucleocapsid Protein (N-protein) is the most abundant protein in coronavirus. The N-protein is a highly immunogenic phosphoprotein, and it is normally very conserved. The N protein of coronavirus is often used as a marker in diagnostic assays.
The renin–angiotensin system (RAS), or renin–angiotensin–aldosterone system (RAAS), is a hormone system that regulates blood pressure and fluid and electrolyte balance, as well as systemic vascular resistance.
The SARS-CoV-2 genome was rapidly sequenced by Chinese researchers. It is an RNA molecule of about 29,811 bases containing 15 genes, including the S gene which codes for a protein located on the surface of the viral envelope (for comparison, our genome is in the form of a double helix of DNA about 3 billion bases in size and contains about 30,000 genes).
Full-genome sequencing and phylogenic analysis indicated that the coronavirus that causes COVID-19 is a betacoronavirus in the same subgenus as the severe acute respiratory syndrome (SARS) virus (as well as several bat coronaviruses), but in a different clade.
A complete genome sequence was obtained for a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strain isolated from an oropharyngeal swab specimen of a Nepalese patient with coronavirus disease 2019 (COVID-19), who had returned to Nepal after traveling to Wuhan, China. The specimen tested positive for SARS-CoV-2 by real-time reverse transcriptase PCR (rRT-PCR) developed in the University of Hong Kong.
Sequencing was done using the Illumina MiSeq system with the Burrows-Wheeler Aligner MEM algorithm (BWA-MEM) 0.7.5a-r405 assembly method. The full genome was amplified directly from the RNA extract from the original specimen using gene-specific primers for open reading frame 1b (ORF1b) and N to produce overlapping PCR products covering the full genome. The new genome sequence was obtained by first mapping reads to a reference SARS-CoV-2 genome using BWA-MEM 0.7.5a-r405 with default parameters to generate the consensus sequence. In addition, the assembly produced by MEGAHIT 1.2.9 (de novo assembly), using default parameters, was used to cross-validate with the reference-based method as an internal control.
The final genome of sequenced SARS-CoV-2 consists of a single, positive-stranded RNA that is 29,811 nucleotides long, broken down as follows: 8,903 (29.86%) adenosines, 5,482 (18.39%) cytosines, 5,852 (19.63%) guanines, and 9,574 (32.12%) thymines.
The coronaviral genome encodes four major structural proteins: the spike (S) protein, nucleocapsid (N) protein, membrane (M) protein, and the envelope (E) protein.
The S protein is responsible for facilitating entry of the CoV into the target cell. It is composed of a short intracellular tail, a transmembrane anchor, and a large ectodomain that consists of a receptor binding S1 subunit and a membrane-fusing S2 subunit.
Sequence analysis of the SARS-CoV-2 S protein genome showed that it was only 75% identical with the SARS-CoV S protein. However, analysis of the receptor binding motif (RBM) in the S protein showed that most of the amino acid residues essential for receptor binding were conserved between SARS-CoV and SARS-CoV-2, suggesting that the 2 CoV strains use the same host receptor for cell entry. The entry receptor utilized by SARS-CoV is Angiotensin-Converting Enzyme 2 (ACE-2).
Angiotensin-Converting Enzyme 2 (ACE-2) is a type I transmembrane metallocarboxypeptidase with homology to ACE, an enzyme long-known to be a key player in the Renin-Angiotensin system (RAS) and a target for the treatment of hypertension. It is mainly expressed in vascular endothelial cells, the renal tubular epithelium, and in Leydig cells in the testes.
ACE-2 is an Entry Receptor for SARS-CoV-2 – researches:
Based on the sequence similarities of the RBM between SARS-CoV-2 and SARS-CoV, several independent research groups investigated if SARS-CoV-2 also utilizes ACE-2 as a cellular entry receptor. Zhou et al. showed that SARS-CoV-2 could use ACE-2 from humans, Chinese horseshoe bars, civet cats, and pigs to gain entry into ACE-2-expressing HeLa cells. Hoffmann et al. reported similar findings for human and bat ACE- Additionally, Hoffmann et al. showed that treating Vero-E6 cells, a monkey kidney cell line known to permit SARS-CoV replication, with an Anti-ACE-2 Antibody (R&D Systems, Catalog # AF933) blocked entry of VSV pseudotypes expressing the SARS-CoV-2 S protein.
PCR analysis revealed that ACE-2 is also expressed in the lung, kidney, and gastrointestinal tract, tissues shown to harbor SARS-CoV. The major substrate for ACE-2 is Angiotensin II. ACE-2 degrades Angiotensin II to generate Angiotensin 1-7, thereby, negatively regulating the renin angiotensin system (RAS).
ACE-2 has also been shown to exhibit a protective function in the cardiovascular system and other organs.
How does virus jump from animals to humans?
How new coronavirus breaks into human cells?
The analysis of public genome sequence data from SARS-CoV-2 and related viruses found no evidence that the virus was made in a laboratory or otherwise engineered.
SARS-CoV-2 is closely related to the original SARS-CoV.
Genetic analysis has revealed that the coronavirus genetically clusters with the genus Betacoronavirus, in subgenus Sarbecovirus (lineage B) together with two bat-derived strains. It is 96% identical at the whole genome level to other bat coronavirus samples (BatCov RaTG13).
In February 2020, Chinese researchers found that there is only one amino acid difference in certain genome sequences between the viruses found in pangolins and those from human patients, implying that pangolins may have been an intermediate host.
Research institutions used this sequencing data to explore the origins and evolution of SARS-CoV-2, they analyzed the genetic template for spike proteins, armatures on the outside of the virus that it uses to grab and penetrate the outer walls of human and animal cells.
More specifically, they focused on two important features of the spike protein: the receptor-binding domain (RBD), a kind of grappling hook that grips onto host cells, and the cleavage site, a molecular can opener that allows the virus to crack open and enter host cells. The scientists found that the receptor-binding domain (RBD) portion of the SARS-CoV-2 spike proteins had evolved to effectively target a molecular feature on the outside of human cells called ACE2, a receptor involved in regulating blood pressure.
The SARS-CoV-2 spike protein was so effective at binding the human cells, in fact, that the scientists concluded it was the result of natural selection and not the product of genetic engineering.
This evidence for natural evolution was supported by data on SARS-CoV-2’s backbone — its overall molecular structure. If someone were seeking to engineer a new coronavirus as a pathogen, they would have constructed it from the backbone of a virus known to cause illness. But the scientists found that the SARS-CoV-2 backbone differed substantially from those of already known coronaviruses and mostly resembled related viruses found in bats and pangolins.
“These two features of the virus, the mutations in the RBD portion of the spike protein and its distinct backbone, rules out laboratory manipulation as a potential origin for SARS-CoV-2”.
Coronaviruses (CoVs), enveloped positive-sense RNA viruses, are characterized by club-like spikes that project from their surface, an unusually large RNA genome, and a unique replication strategy.
In a phylogenetic analysis of 103 strains of SARS-CoV-2 from China, two different types of SARS-CoV-2 were identified, designated type L (accounting for 70 percent of the strains) and type S (accounting for 30 percent). The clinical implications of these findings are uncertain.
Over the past 50 years the emergence of many different coronaviruses that cause a wide variety of human and veterinary diseases has occurred. It is likely that these viruses will continue to emerge and to evolve and cause both human and veterinary outbreaks owing to their ability to recombine, mutate, and infect multiple species and cell types.
SARS-CoV-2, a single-stranded RNA-enveloped virus, targets cells through the viral structural spike (S) protein that binds to the angiotensin-converting enzyme 2 (ACE2) receptor. Following receptor binding, the virus particle uses host cell receptors and endosomes to enter cells. A host type 2 transmembrane serine protease, TMPRSS2, facilitates cell entry via the S protein. Once inside the cell, viral polyproteins are synthesized that encode for the replicase-transcriptase complex. The virus then synthesizes RNA via its RNA-dependent RNA polymerase. Structural proteins are synthesized leading to completion of assembly and release of viral particles. These viral lifecycle steps provide potential targets for drug therapy.
Coronaviruses (CoVs) are the largest group of viruses belonging to the Nidovirales order, which includes Coronaviridae, Arteriviridae, and Roniviridae families. The Coronavirinae comprise one of two subfamilies in the Coronaviridae family, with the other being the Torovirinae. The Coronavirinae are further subdivided into four groups, the alpha, beta, gamma and delta coronaviruses. The viruses were initially sorted into these groups based on serology but are now divided by phylogenetic clustering.
All viruses in the Nidovirales order are enveloped, non-segmented positive-sense RNA viruses. They all contain very large genomes for RNA viruses, with Coronavirinae having the largest identified RNA genomes, containing approximately 30 kilobase (kb) genomes.
Other common features within the Nidovirales order include:
The clinical spectrum of SARS-CoV-2 infection appears to be wide, encompassing asymptomatic infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure and even death.
Asymptomatic (their frequency is unknown) or develop symptoms such as fever, cough, fatigue, shortness of breath, or muscle pain.
A WHO review of 55,924 laboratory-confirmed cases in China indicated the following typical signs and symptoms:
Other, less common symptoms have included
Further development can lead to severe pneumonia, acute respiratory distress syndrome, sepsis, septic shock, and death. Some of those infected may be asymptomatic, returning test results that confirm infection but show no clinical symptoms, so researchers have issued advice that individuals with close contact to confirmed infected patients should be closely monitored and examined to rule out infection.
There are no specific clinical features that can yet reliably distinguish COVID-19 from other viral respiratory infections.
Spectrum of illness severity
According to the WHO, recovery time appears to be around two weeks for mild infections and three to six weeks for severe disease.
Covid-19 symptoms vs. other viruses
| Symptoms | Coronavirus | Cold | Flu |
| Fever | Common | Rare | Common |
| Cough | Sometimes | Sometimes | Common |
| Sneezing | No | Common | No |
| Aches and Pain | Sometimes | Common | Common |
| Runny or stuffy nose | Rare | Common | Sometimes |
| Sore throat | Sometimes | Common | Sometimes |
| Diarrhea | Rare | No | Sometimes |
| Headaches | Sometimes | Rare | Common |
| Shortness of breath | Sometimes | No | No |
The level and duration of infectious virus replication are important factors in assessing the risk of transmission and guiding decisions regarding the isolation of patients. Because coronavirus RNA detection is more sensitive than virus isolation, most studies have used qualitative or quantitative viral RNA tests as a potential marker for infectious coronavirus.
Whereas the duration of SARS-CoV-2 RNA detection has not been well characterised, in one study, it was found that the detectable SARS-CoV-2 RNA persisted for a median of 20 days in survivors and that it was sustained until death in non-survivors.
Viral load in nasal and throat swabs
The WHO has published several RNA testing protocols for SARS-CoV-2, the virus that causes COVID-19, with the first issued on 17 January. Testing uses real time reverse transcription polymerase chain reaction (rRT-PCR). The test can be done on respiratory or blood samples. Results are generally available within a few hours to days.
Signs of pneumonia may precede confirmation of COVID-19 infection through RT-PCR.
An alternative method of diagnosis is based on clinical presentation such as looking for visual signature patterns of COVID-19 in CT scans of the lungs.
Imaging findings – Chest CT in patients with COVID-19 most commonly demonstrates ground-glass opacification with or without consolidative abnormalities, consistent with viral pneumonia.
Case series have suggested that chest CT abnormalities are more likely to be bilateral, have a peripheral distribution, and involve the lower lobes. Less common findings include pleural thickening, pleural effusion, and lymphadenopathy.
In one report of 21 patients with laboratory-confirmed COVID-19 who did not develop severe respiratory distress, lung abnormalities on chest imaging were most severe approximately 10 days after symptom onset.
However, chest CT abnormalities have also been identified in patients prior to the development of symptoms and even prior to the detection of viral RNA from upper respiratory specimens.
Even patients with asymptomatic infection may have objective clinical abnormalities. As an example, in a study of 24 patients with asymptomatic infection who all underwent chest computed tomography (CT), 50 percent had typical ground-glass opacities or patchy shadowing, and another 20 percent had atypical imaging abnormalities. Five patients developed low-grade fever, with or without other typical symptoms, a few days after diagnosis.


The goal of all vaccines is to elicit an immune response against an antigen so that when the individual is again exposed to the antigen, a much stronger secondary immune response will result. Vaccines contain the same antigens that are found on pathogens that cause the associated disease, but exposure to the antigens in vaccines is controlled. By priming the immune system through vaccination, when the vaccinated individual is later exposed to the live pathogens in the environment, the immune system can destroy them before they can cause disease.
Thus, there are two ways of acquiring immunity to a pathogen – by natural infection and by vaccination. Natural infections and vaccines produce a very similar end result – immunity – but the person who receives a vaccine does not endure the illness and its potential life-threatening complications. The very low risk of an adverse event caused by a vaccine greatly outweighs the risk of illness and complications caused by natural infection.
There are no vaccines currently available. Several organisations around the world are developing vaccines, using several different methods.
The most effective long-term strategy for prevention of future outbreaks of this virus would be the development of a vaccine providing protective immunity. However, a minimum of 12 to 18 months would be required before widespread vaccine deployment.
As of 8 April 2020, the global COVID-19 vaccine R&D landscape includes 115 vaccine candidates, of which 78 are confirmed as active and 37 are unconfirmed (development status cannot be determined from publicly available or proprietary information sources). Of the 78 confirmed active projects, 73 are currently at exploratory or preclinical stages. The most advanced candidates have recently moved into clinical development, including mRNA-1273 from Moderna, Ad5-nCoV from CanSino Biologicals, INO-4800 from Inovio, and LV-SMENP-DC and pathogen-specific aAPC from Shenzhen Geno-Immune Medical Institute. Numerous other vaccine developers have indicated plans to initiate human testing in 2020.
A feature of the vaccine development landscape for COVID-19 is the range of technology platforms being evaluated, including nucleic acid (DNA and RNA), virus-like particle, peptide, viral vector (replicating and non-replicating), recombinant protein, live attenuated virus and inactivated virus approaches.
Clinical – phase vaccine candidates for CoVid-19 – Nature Reviews Drug Discovery
DRAFT landscape of COVID-19 candidate vaccines – 23 April 2020 World Health Organization (WHO)
World Health Organization (WHO) for information purposes only concerning the 2019-2020 global of the novel coronavirus.
Link: https://www.who.int/blueprint/priority-diseases/key-action/draft-landscape-COVID-19-candidate-vaccines-23-April-2020.pdf

The pandemic of coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents an unprecedented challenge to identify effective drugs for prevention and treatment.
There are no specific antiviral medications, though development efforts are underway. Attempts to relieve the symptoms may include taking regular (over-the-counter) cold medications, drinking fluids, and resting. Depending on the severity, oxygen therapy, intravenous fluids, and breathing support may be required. Some countries require people to report flu-like symptoms to their doctor, especially if they have visited mainland China.
The viral lifecycle steps provide potential targets for drug therapy. Promising drug targets include nonstructural proteins (eg, 3-chymotrypsin-like protease, papain-like protease, RNA-dependent RNA polymerase), which share homology with other novel coronaviruses (nCoVs). Additional drug targets include viral entry and immune regulation pathways.
This review of proposed drugs is by necessity selective. A review of the American Chemical Society, analyzed scientific data related to therapeutic agents and vaccines in human coronaviruses since 2003, reported more than 130 patents and more than 3000 potential small molecule drug candidates with potential activity against human coronaviruses, identified more than 500 patents for biologic agents with activity against coronaviruses including therapeutic antibodies, cytokines, RNA therapies, and vaccines. Another preprint analysis of SARS-CoV-2–human protein-protein interaction maps identified 332 high-confidence protein-protein interactions, yielding 66 candidate druggable human proteins or host factors targeted. This large amount of potential agents will hopefully yield more candidate therapeutics in the race to find effective treatments or preventive strategies against COVID-19.
Adjunctive Therapies
Limited role of glucocorticoids – The WHO and CDC recommend glucocorticoids not be used in patients with COVID-19 pneumonia unless there are other indications (eg, exacerbation of chronic obstructive pulmonary disease). Glucocorticoids have been associated with an increased risk for mortality in patients with influenza and delayed viral clearance in patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Although they were widely used in management of severe acute respiratory syndrome (SARS), there was no good evidence for benefit, and there was persuasive evidence of adverse short- and long-term harm.
Prostaglandins are produced within the body’s cells by the enzyme cyclooxygenase (COX). There are two COX enzymes, COX-1 and COX-2. Both enzymes produce prostaglandins that promote inflammation, pain, and fever. However, only COX-1 produces prostaglandins that support platelets and protect the stomach. Nonsteroidal anti-inflammatory drugs (NSAIDs) block the COX enzymes and reduce prostaglandins throughout the body. As a consequence, ongoing inflammation, pain, and fever are reduced. Since the prostaglandins that protect the stomach and support platelets and blood clotting also are reduced, NSAIDs can cause ulcers in the stomach and promote bleeding.
Nonsteroidal anti-inflammatory drug, a medication that is commonly prescribed or purchased over the counter to treat the inflammation associated with conditions such as arthritis, tendonitis, and bursitis. Examples of NSAIDs include aspirin, indomethacin (brand name: Indocin), ibuprofen (brand name: Motrin), naproxen (brand name: Naprosyn), piroxicam (brand name: Feldene), and nabumetone (brand name: Relafen). People who take certain NSAIDs may have a higher risk of having a heart attack or a stroke than people who do not take these medications. This risk may be higher for people who take NSAIDs for a long time. Other major side effects of NSAIDs are gastrointestinal problems. Some 10 to 50 percent of patients are unable to tolerate NSAID treatment because of these side effects, which include abdominal pain, diarrhea, bloating, heartburn, and upset stomach.
Uncertainty about NSAID use – Some clinicians have suggested the use of non-steroidal anti-inflammatory drugs (NSAIDs) early in the course of disease may have a negative impact on disease outcome. These concerns are based on anecdotal reports of a few young patients who received NSAIDs early in the course of infection and experienced severe disease, as well as the theoretic concern that the anti-inflammatory properties associated with NSAIDs could have a negative impact on the patient’s immune response. In light of these concerns, some providers are using acetaminophen in place of NSAIDs for reduction of fever; however, the European Medicines Agency (EMA) and the WHO do not recommend that NSAIDs be avoided when clinically indicated.
Tocilizumab – Treatment guidelines from China’s National Health Commission include the IL-6 inhibitor tocilizumab for patients with severe COVID-19 and elevated IL-6 levels.
It has been used in small series of severe COVID-19 cases with early reports of success. A report of 21 patients with COVID-19 showed receipt of tocilizumab, 400 mg, was associated with clinical improvement in 91% of patients as measured by improved respiratory function, rapid defervescence, and successful discharge, with most patients only receiving 1 dose.
Sarilumab, another IL-6 receptor antagonist approved for RA, is being studied in a multicenter, double-blind, phase 2/3 trial for hospitalized patients with severe COVID-19. Other monoclonal antibody or immunomodulatory agents in clinical trials in China include bevacizumab (anti–vascular endothelial growth factor medication), fingolimod (immunomodulator approved for multiple sclerosis), and eculizumab (antibody inhibiting terminal complement).
Interferon-α and -β
Interferon alfa belongs to the category of therapies called biologic response modifiers (BRM), also called immunotherapy. This is a type of treatment that mobilizes the body’s immune system to fight cancer. The therapy mainly consists of stimulating the immune system to help it do its job more effectively.
Interferon-β is a complex immunomodulator that exerts a wide range of effects, including inhibition of leukocyte proliferation, T-cell trafficking, and antigen presentation; additionally, it may reduce the levels of proinflammatory cytokines and increase those of anti-inflammatory cytokines.
Interferon-α and -β have been studied for nCoVs, with interferon-β demonstrating activity against MERS. Most published studies reported results of therapy combined with ribavirin and/or lopinavir/ritonavir. Similar to other agents, delayed treatment may limit effectiveness of these agents. Given conflicting in vitro and animal data and the absence of clinical trials, the use of interferons to treat SARS-CoV-2 cannot currently be recommended.
Other immunomodulatory agents traditionally used for noninfectious indications demonstrate in vitro activity or possess mechanisms purported to inhibit SARS-CoV-2, including, but not limited to,
Baricitinib, an orally bioavailable inhibitor of Janus kinases 1 and 2 (JAK1/2), with potential anti-inflammatory, immunomodulating and antineoplastic activities. Baricitinib may induce apoptosis and reduce proliferation of JAK1/2-expressing tumor cells. JAK kinases are intracellular enzymes involved in cytokine signaling, inflammation, immune function and hematopoiesis; they are also upregulated and/or mutated in various tumor cell types.
Imatinib, a small molecule kinase inhibitor used to treat certain types of cancer. It is currently marketed as its mesylate salt, imatinib mesilate (INN). It is occasionally referred to as CGP57148B or STI571 (especially in older publications). It is used in treating chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and a number of other malignancies.
Dasatinib, Dasatinib Anhydrous is an orally bioavailable synthetic small molecule-inhibitor of SRC-family protein-tyrosine kinases. Dasatinib binds to and inhibits the growth-promoting activities of these kinases. Apparently because of its less stringent binding affinity for the BCR-ABL kinase, dasatinib has been shown to overcome the resistance to imatinib of chronic myeloid leukemia (CML) cells harboring BCR-ABL kinase domain point mutations. SRC-family protein-tyrosine kinases interact with variety of cell-surface receptors and participate in intracellular signal transduction pathways; tumorigenic forms can occur through altered regulation or expression of the endogenous protein and by way of virally-encoded kinase genes.
Cyclosporine, is a calcineurin inhibitor known for its immunomodulatory properties that prevent organ transplant rejection and treat various inflammatory and autoimmune conditions. It is isolated from the fungus Beauveria nivea.
Nitazoxanide, the antiprotozoal activity of nitazoxanide is believed to be due to interference with the pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reaction which is essential to anaerobic energy metabolism. The antiviral activity, immunomodulatory effects, and safety profile of nitazoxanide warrant its further study as a treatment option for SARS-CoV-2.
Camostat mesylate, an approved agent in Japan for the treatment of pancreatitis, prevents nCoV cell entry in vitro through inhibition of the host serine protease, TMPRSS2.
Chloroquine and hydroxychloroquine have a long-standing history in the prevention and treatment of malaria and the treatment of chronic inflammatory diseases including systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Dosing of chloroquine to treat COVID-19 has consisted of 500 mg orally once or twice daily. However, a paucity of data exists regarding the optimal dose to ensure the safety and efficacy of chloroquine.Both agents can cause rare and serious adverse effects (<10%), including QTc prolongation, hypoglycemia, neuropsychiatric effects, and retinopathy. Use of chloroquine and hydroxychloroquine in pregnancy is generally considered safe. 7-9-10-11-12- 15- 17 – 18- 39-
If chloroquine is shown to be effective against SARS-CoV-2, it will not be via the same mechanism by which the drug functions as an antimalarial. That’s because malaria is caused not by a virus but by a microparasite Plasmodium falciparum. Chloroquine makes it toxic for the parasite to digest its host’s hemoglobin. The clinical usefulness of chloroquine, and in some recent cases of quinine as well, has been much reduced by the evolution and spread of chloroquine resistant malaria parasites.
Chloroquine might have entirely different effects against a virus, such as, for example, disrupting the virus’s ability to enter a cell by inhibiting glycosylation of host receptors, proteolytic processing, and endosomal acidification. These agents also have immunomodulatory effects through attenuation of cytokine production and inhibition of autophagy and lysosomal activity in host cells.
A news briefing from China reported chloroquine was successfully used to treat a series of more than 100 COVID-19 cases resulting in improved radiologic findings, enhanced viral clearance, and reduced disease progression. However, the clinical trial design and outcomes data have not yet been presented or published for peer review, preventing validation of these claims.
Both chloroquine and hydroxychloroquine have been reported to inhibit SARS-CoV-2 in vitro, although hydroxychloroquine appears to have more potent antiviral activity.
Use of chloroquine is included in treatment guidelines from China’s National Health Commission and was reportedly associated with reduced progression of disease and decreased duration of symptoms. However, primary data supporting these claims have not been published.
Other published clinical data on either of these agents are limited. Examples:
Despite the limited clinical data, given the relative safety of short-term use of hydroxychloroquine (with or without azithromycin), the lack of known effective interventions, and the in vitro antiviral activity, some clinicians think it is reasonable to use one or both of these agents in hospitalized patients with severe or risk for severe infection, particularly if they are not eligible for other clinical trials.
Remdesivir is a monophosphate prodrug with activity against RNA viruses, such as Coronaviridae and Flaviviridae.
Remdesivir is a prodrug that metabolizes into its active form GS-441524 an adenosine nucleotide analog that interferes with the action of viral RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), causing a decrease in viral RNA production. It was unknown whether it terminates RNA chains or causes mutations in them.
It was previously tested in humans with Ebola virus disease and has shown promise in animal models for treating Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), which are caused by other coronaviruses.
Several randomized trials are underway to evaluate the efficacy of remdesivir for moderate or severe COVID-19. The safety and pharmacokinetics of remdesivir were evaluated in single- and multiple-dose phase 1 clinical trials. The current dose under investigation is a single 200-mg loading dose, followed by 100-mg daily infusion. Intravenous infusions between 3 mg and 225 mg were well-tolerated without any evidence of liver or kidney toxicity.
Currently, remdesivir is a promising potential therapy for COVID-19 due to its broad-spectrum, potent in vitro activity against several nCoVs, including SARS-CoV-2.
Favipiravir, previously known as T-705, is a prodrug of a purine nucleotide, favipiravir ribofuranosyl-5′-triphosphate. The active agent inhibits the RNA polymerase, halting viral replication. Limited clinical experience has been reported supporting the use of favipiravir for COVID-19.
Doses at the higher end of the dosing range should be considered for the treatment of COVID-19. A loading dose is recommended (2400 mg to 3000 mg every 12 hours × 2 doses) followed by a maintenance dose (1200 mg to 1800 mg every 12 hours). The half-life is approximately 5 hours.
Lopinavir-ritonavir – The combination of lopinavir and ritonavir is used with other medications to treat human immunodeficiency virus (HIV) infection. Lopinavir and ritonavir are in a class of medications called protease inhibitors. They work by decreasing the amount of HIV in the blood. When lopinavir and ritonavir are taken together, ritonavir also helps to increase the amount of lopinavir in the body so that the medication will have a greater effect. Although lopinavir and ritonavir will not cure HIV, these medications may decrease the chance of developing acquired immunodeficiency syndrome (AIDS) and HIV-related illnesses such as serious infections or cancer.
This combined protease inhibitor, lopinavir and ritonavir, has in vitro activity against the SARS-CoV and appears to have some activity against MERS-CoV in animal studies . Although the use of this agent for treatment of COVID-19 has been described in case reports, there was no difference in time to clinical improvement or mortality at 28 days in a randomized trial of 199 patients with severe COVID-19 given lopinavir-ritonavir (400/100 mg) twice daily for 14 days in addition to standard care versus those who received standard of care alone.
The most commonly used and studied lopinavir/ritonavir dosing regimen for COVID-19 treatment is 400 mg/100 mg twice daily for up to 14 days. Adverse effects of lopinavir/ritonavir include gastrointestinal distress such as nausea and diarrhea (up to 28%) and hepatotoxicity (2%-10%).
Other antiretrovirals, including protease inhibitors and integrase strand transfer inhibitors, were identified by enzyme activity screening as having SARS-CoV-2 activity.
Ribavirin
Ribavirin, a guanine analogue, inhibits viral RNA-dependent RNA polymerase. Its activity against other nCoVs makes it a candidate for COVID-19 treatment. However, its in vitro activity against SARS-CoV was limited and required high concentrations to inhibit viral replication, necessitating high-dose (eg, 1.2 g to 2.4 g orally every 8 hours) and combination therapy. Ribavirin causes severe dose-dependent hematologic toxicity. Ribavirin is also a known teratogen and contraindicated in pregnancy.
Oseltamivir
Oseltamivir, a neuraminidase inhibitor approved for the treatment of influenza, has no documented in vitro activity against SARS-CoV-2. This agent has no role in the management of COVID-19 once influenza has been excluded.
Umifenovir
Umifenovir (also known as Arbidol) is a more promising repurposed antiviral agent with a unique mechanism of action targeting the S protein/ACE2 interaction and inhibiting membrane fusion of the viral envelope. The current dose of 200 mg orally every 8 hours for influenza is being studied for COVID-19 treatment. A nonrandomized study of 67 patients with COVID-19 showed that treatment with umifenovir for a median duration of 9 days was associated with lower mortality rates (0% [0/36] vs 16% and higher discharge rates compared with patients who did not receive the agent.
Immunoglobulin Therapy
Another potential adjunctive therapy for COVID-19 is the use of convalescent plasma or hyperimmune immunoglobulins. The rationale for this treatment is that antibodies from recovered patients may help with both free virus and infected cell immune clearance.
National response measures have included containment measures such as lockdowns, quarantines, and curfews.
Type in your illness
Premilife gives energy to the human body that increases the chances of winning diseases (which diseases? more than 700!) - the means - homeopathic remedies
Darle a tu cuerpo las herramientas que necesita para vencer a la enfermedad/es.
Tratar con la enfermedad puede agotaros psicológica y emocionalmente. Por eso, para que el tratamiento tenga éxito, también necesitamos prepararos y ayudaros a defenderos contra estos efectos mentales.
Los tratamientos exitosos atacan al problema de raíz. Hay muchas maneras de simplemente tratar el dolor o los síntomas obvios. Nosotros intentamos tratar la causa de la enfermedad, y la capacidad de la enfermedad para cambiar vuestro cuerpo. Nos centramos en la energía del cuerpo, y en las energías de la enfermedad.

Desde 1980, el Dr. Kotliroff ha ayudado a miles de pacientes de todo el mundo con sus fórmulas únicas y ahora tú también tendrás la oportunidad de oro de asistir a su evento online en directo y de comprender tu problema, así como de aprender de él sobre su excepcional fórmula. Va a ser un evento online en directo especial y para entender su fórmula secreta, ¡únete a nosotros!
Encuentra tu (s) enfermedad (s) escribiendo el nombre de tu enfermedad.
HOW you can deal with your serious issues that arise out of your diseases WITHOUT feeling depressed or losing faith.
EVEN IF you have lost all the hopes, you can make it happen!
Give your body the tools to defeat your disease/s.
Dealing with illness can drain you psychologically and emotionally. And so for treatment to be successful, we also need to prepare you for and help to defend you against these mental effects.
Successful treatments target the root problem. There are many ways just to treat pain, or obvious symptoms. We’re looking to treat the cause of the disease, and the disease’s ability to change your body. We’re focusing on the energy of the body, and the energies of disease.

Since 1980, Dr. Kotliroff has helped thousands of patients all over the globe with his one-of-a-kind formulas and now you will also have the golden opportunity to come to his online live event and understand your problem, and also learn from him about his remarkable formula. It is going to be a special online live event and to understand his secret formula, come and join us!
Find your disease/s by typing your disease name.